With its first foray into medical devices, Schweitzer Engineering Laboratories hopes to help children with autism spectrum disorder be diagnosed and treated earlier in life.
The manufacturer based in Washington state announced recently that it’s moving forward with efforts to bring a handheld autism medical technology device to clinics across the country. In 2024, it acquired the intellectual property and hired the researcher who developed it at Washington State University Spokane.
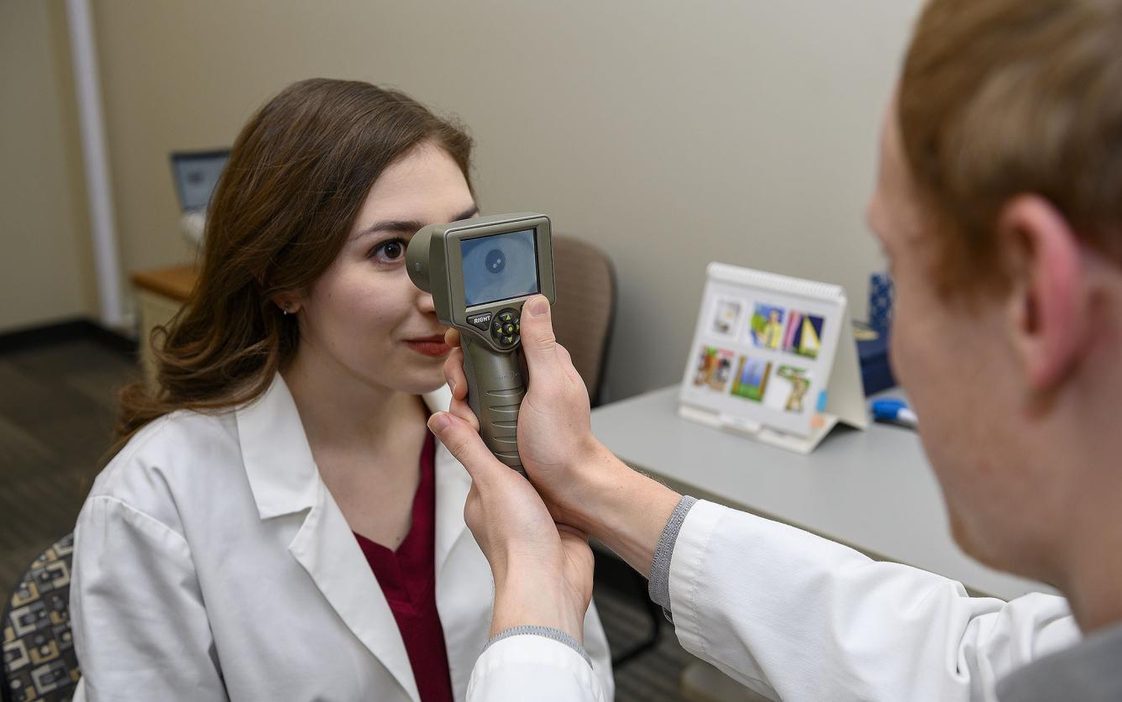
While yet to receive federal approval, the device can detect atypical reactions to light in a child’s pupils, which research has shown to bean indicator of autism.
Autism is a developmental disability that affects about 1 in 36 children, according to the Centers for Disease Control and Prevention. Although there are differences on the spectrum, autism often causes problems with social communication and interaction and restricted or repetitive behaviors or interests.
It does not provide a clear-cut diagnosis, but the data the device gathers through analyzing pupil activity would be an invaluable tool in the screening process for clinicians, said Georgina Lynch, the researcher who developed a prototype while an associate professor at the WSU Elson S. Floyd College of Medicine.
Schweitzer Engineering purchased the technology in 2024 and hired Lynch to oversee the medical devices team responsible for its development.
“We very much believe that there is a need for objective, physical markers that are associated with autism to help guide the screening process,” Lynch said.
Lynch said the 6-inch-wide device samples at a rate of 80 frames per second and takes pictures of the eye as it responds to light.
She explained that her research and peers at other institution shave shown that the pupillary light reflex provides a sort of window into a child’s brain activity. The efficiency of the nerves for the light reflex indirectly allows clinicians to measure the efficiency of the cranial nerves in the brain stem.
Lynch explained the device analyzes brainstem activity via the pupillary reflex, offering a new understanding of autism. She envisions it as a routine check-up tool, like blood pressure measurement, enabling earlier identification of potential autism. This objective data, unlike current subjective methods, could lead to earlier diagnoses and intervention, improving outcomes. Lynch believes the device could also destigmatize autism by making screening a routine part of care.
Dawn Sidell, director of the Northwest Autism Center, emphasized the critical role of early intervention, noting the "exponential benefits" of earlier access to services. She stressed the value of objective data in screening. Lynch and SEL are now navigating the FDA approval process, anticipating it will take time. Lynch expressed excitement about the potential of this academic-industry partnership to help children.
SEF’s Autism Independence Project helps high-functioning students on the Autism Spectrum by increasing social skills and expanding their meaningful life aspirations. To learn more, visit our website.
The full article about this device is available on the DisabilityScoop website.